|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
Early Signs of Equine
Protozoal Myeloencephalitis
Siobhan P. Ellison, DVM, PhD
Tom Kennedy, PhD
Karen K. Brown, PhD
Department of Nutrition
Faculty of Veterinary Medicine
USA
Key
words: Equine myeloencephalitis, Sarcocystis neurona, horses, central
nervous system
Abstract
The
early signs of Sarcocystis. neurona encephalitis after experimental infection in 6
horses are described. Blinded examiners determined scores for ataxia,
dysmetria, paresis, and spaciticy as part of a gait assessment score.
Other signs of infection noted by blinded examiners were decreased tongue
tone, facial paresis, increased or decreased skin sensation, muscle
wasting, weakness on tail pull, and lameness. Signs other than
ataxia were apparent to blinded examiners by 30 days after challenge and remained
until the end of the observation period. Other signs of acute infection
seen by non-blinded observers included behavior change, drooling, dropped
feed, and transient cranial nerve deficits. A Grade 2 ataxia seen on
2 blinded examinations 30 days apart was used as a definition of equine
protozoal myeloencephalitis (EPM) and was seen in 3 horses, and lameness
was seen in 5 horses. Acute early signs of infection were present before
ataxia in all horses that progressed to EPM or became lame. An observation
period of 90 days was not long enough to observe EPM in one horse.
Introduction
Protozoa gained attention as an important cause of neurologic lameness in the horse in 1974 with the recognition of Toxoplasma gondii-like organisms in the central nervous systems (CNS) of diseased horses.1-3 In 1991, protozoa from the spinal cord of a horse were isolated by continuous culture and named Sarcocystis neurona.4-5 Since the identification of S. neurona as the etiologic agent of equine protozoal myeloencephalitis (EPM), the life cycle between natural host and several intermediate hosts has been achieved in the laboratory.6-7 The premortem diagnosis of EPM includes lameness or neurologic disease that is due to another cause and presence of antibodies to S. neurona in the cerebral spinal fluid. Although the presence of antibody in the spinal fluid of horses with signs of neurologic abnormality measured by immunoblot is considered an aid for diagnosis of EPM, it is recommended that one consider the prevalence of disease in the area at a given time.
Probably, many more horses have marginal clinical
signs of EPM that are never diagnosed but remain part of the large uncharacterized
group of "poor performers." The early signs of infection by S. neurona are
unrecognized because experimental reproduction of the disease has failed.8
Experimental models of EPM by dosing horses with tens of millions of
sporocysts derived from the opossum have not resulted in reproducible
disease that includes isolation of the organism from neurologic tissue.9
The Ellison model of EPM uses infected lymphocytes to facilitate parasite
entry into the CNS that has resulted in clinical signs of EPM and isolation
of the organism from the spinal tissues.
A gut phase of infection is followed by parasitemia in the natural intermediate host for many Sarcocystis species.10-13 The ingestion of low-virulent S. neurona sporocysts shed by the opossum is thought to lead to a biphasic disease characterized by an initial, generalized phase (resulting in production of serum antibodies) with vascular distribution to somatic cells. In some animals, a neurologic stage by which encephalitis with CNS damage from parasites or secondary inflammation to the presence of parasites can develop. Although the horse produces antibodies, immunity to S. neurona is probably T cell mediated, and increasing evidence suggest a critical role of cytokines for an effective immune response.14,15 Researchers took advantage of mice lacking the interferon (IFN)-gamma-receptor to produce S. neurona encephalitis in an experimental host and have used this animal as a model to study EPM.16 Inactivation of the IFN-gamma-receptor rendered mice highly susceptible to S. neurona, and they developed sarcocystis encephalitis. Some naturally infected horses develop encephalitis but do not have an identified deficient IFN-gamma receptor. Studying experimental disease in immunocompetent horses is important to define the pathophysiology of disease. In more commonly recognized equine disease, the signs include ataxia, paresis, lameness, and muscle wasting; however, early signs of disease have not been described. This report describes the early signs of S. neurona infections observed in 6 experimentally challenged horses observed for 90 days.
Materials and Methods
Six horses were selected based on normal gait analysis, normal cervical radiographs, absence of EHV antibodies, and absence of antibodies to S. neurona measured by enzyme-linked immunosorbent assay (ELISA) to recombinant SAG1 (rSAG1), the major surface antigen of S. neurona, in CSF.17,18 Horses selected represented both genders, ranged from 9 months to 15 years old, and were Thoroughbred, Quarter Horse, Mustang, or mixed breed. The animals were housed in a Bahia grass paddock and fed pelleted commercial equine ration and alfalfa hay. A number on the halter and a neck strap identified the animals. Water was provided free choice. Horses were challenged with S. neurona and monitored daily.19 Briefly, lymphocytes were obtained from each animal and the lymphocytes were infected in vitro with merozoites of S. neurona. Approximately 6,000 infected autologous lymphocytes were given intravenously to each horse for 14 days. Serum and CSF samples were obtained before infection and at approximately 30, 60, and 90 days.
Clinical signs of ataxia and other clinical signs of neurologic disease were observed by veterinarians blinded to the study at 4 times: at selection, at 30 days, at 60 days, and at 90 days. Ataxia was scored for each limb and recorded as a Grade 0 (normal), Grade 1 (just detected at a normal gait), Grade 2 (deficit easily detected and exaggerated by backing, turning, swaying, loin pressure, and neck extension), or Grade 3 (deficit very prominent on walking, turning, loin pressure, or neck extension). A gait score was determined by adding the ataxia score for each leg assessed for ataxia, paresis, spasticity, and dysmetria. Other signs of infection were recorded and included decreased tongue tone, presence of facial paresis, increased or decreased skin sensation, muscle wasting, weakness on tail pull, and lameness. Each sign noted was scored as 1, and the cumulative score was used as the score for other signs. An antibody index (AI) for S. neurona was calculated using the formula antibody quotient divided by albumin quotient. The antibody quotient was calculated from the CSF titer multiplied by 1,000 divided by serum titer (the titer was the reciprocal of the last dilution with a positive A405 determined by SAG1 ELISA) divided by serum albumin multiplied by 1,000. The AI was considered significant if the calculated value was greater than 0.7. The SAG1 ELISA is an indirect ELISA test that detects antibodies to recombinant S. neurona SAG1 and is considered significant at a titer of 100 for serum.18
Results
Initially, behavior changed, and horses were slower to respond to feed and less eager to enter the feeding pens and they ate feed slowly. Horses were less aggressive in the herd. Horses drooled and had decreased tongue tone, resulting in dropped feed. Mild and transient unilateral facial paresis with slack lips (inability to display flamen response) and sometimes eyelid paresis was seen. Weakness, limb paresis, and ataxia were seen. Muscle wasting was seen in 2 animals that showed signs of paresis and ataxia. An absent "slap test," palpation of the cricoaritinoid muscle while slapping the opposite wither, was noted in some animals. As horses became weak and ataxic, some horses stood in a "parked out" stance or were recumbent more than 50% of the time. The cranial nerve signs were often transient and tended to improve as the disease progressed. The immunoconversion of CSF from negative to positive was seen in all horses.
Data recorded by blinded observers are shown in Figures 1 through 6. Observatons recorded by the author (Ellison) is shown in Figure 7. An increase in CSF antibodies (line on graphs) coincided with acute signs of disease by 30 days after challenge (5 of 6) or by 90 days (1 of 6). A Grade 2 ataxia or lameness (shown in a table under the graph) was present in 5 of 6 horses and was present by 30 days in 3 horses, 60 days in 2 horses, and absent in 1 horse. The presence of lameness or ataxia accompanied or followed the acute signs of infection, shown as a bar on the graphs. All horses produced serum antibodies detected by SAG1 ELISA; the titer is shown in a table under the graph.
In horse 1 (Fig. 1), a serum antibody titer of 400 coincided with the appearance of specific CSF antibodies, AI 0.15, at 30 days after challenge. The AI increased to 1.1 and decreased to 0 while the serum antibodies increased to 1,600 by 90 days. Ataxia was not seen, but early signs of infection were seen by the end of the observation period. In this horse, the period of observation was not sufficient to determine if the infection would progress to EPM or the disease would resolve.
In horse 2 (Fig. 2), a serum antibody titer of 200, present at 60 days, followed the appearance of specific CSF antibodies, AI 4.4, at 30 days after challenge. The AI decreased over the next 60 days. During the observation period the horse was not ataxic but was lame, the acute signs became progressively worse during the observation period. In this horse, the period of observation was not sufficient to determine if the gait deficits would progress to ataxia however, the horse was progressively worse during the observation period.
Horse 3 produced serum and CSF antibodies at 60 days after challenge although the AI was only 0.73; the early signs of lameness and other cranial nerved deficits were noted at 30 days. The deficits noted became progressively worse during the observation period. Horse 4 produced serum antibodies and had early signs of disease at 30 days and produced CSF antibodies, AI 7.01, by 60 days. Ataxia was observed at 60 days. The horse was scored a Grade 2 for EPM at 90 days, but the total gait score had decreased because ataxia, paresis, spasticity, and dysmetria was not noted in all limbs. Lameness and ataxia were present at the end of the observation period and gait deficits had been recorded for 60 days.
Horse 5 had serum
antibodies at 60 days; however, CSF antibodies, AI 17.2, were detected
at 30 days after challenge. Early signs, including lameness without
ataxia, were present 30 days after challenge. The ataxia became progressively
worse during the observation period. Horse 6 had serum antibodies, titer
200, at selection and the antibody titer increased to 1,600 by the end
of the observation period. Detection of CSF antibodies, AI 3.3, at 60
days followed early clinical signs of EPM that included lameness and
ataxia that were detected at 30 days after challenge.
Discussion
Equine protozoal myeloencephalitis is an important infectious disease of horses in this country. Significant advances for both management and treatment strategies will not be made without improvements in the recognition of acute disease in the horse. Our results, when taken together, identify early signs present in the horse after S. neurona has crossed the blood brain barrier. We identified the presence of antibodies in the CSF to S. neurona in response to challenge using an indirect ELISA that used rSAG1 as antigen. A specific measure of S. neurona antibodies in the CSF was calculated and expressed as an antibody index. It was interesting to note that by 90 days the CSF antibodies had decreased in all the horses.
Simultaneous with the presence of CSF antibodies, we noted subtle cranial nerve deficits. The involvement of cranial nerves V (facial sensation), VII (facial symmetry), IX (swallow), and X (swallow) were noted early and in some cases transient. Signs seen before the first blinded examination included dropping feed, decreased tongue tone, facial paresis, mentation change, generalized weakness, and lameness. Lameness was seen as soon as 10 days after challenge, and muscle atrophy in the paretic limb was seen as early as day 24. Generalized weakness was difficult to measure but was apparent in the animals demeanor, lethargic attitude when led, preference for a parked out stance, and, in some cases, recumbence. Blinded evaluators detected weakness using a tail pull at a walk. The presence of the acute signs preceded lameness and ataxia in 4 horses and coincided with lameness and ataxia in one horse. In one horse, mild signs were present at the end of the observation period, but the horse was not ataxic. Therefore, a diagnosis of EPM was not made. Five of the 6 challenge horses produced serum antibodies that remained detectable during the observed period. All horses had early signs of disease, including lameness (5 of 6); however, the lameness was not always evaluated as having a component that was described as ataxic, paretic, spastic, or dysmetric. Therefore, the animal was not considered by the evaluators to have EPM at that examination. The determination of ataxia was only made in 3 of the horses.
Our results indicate that EPM can manifest as long as 90 days after the parasite has crossed the blood-brain barrier (data not shown), but in most of the challenged horses evidence of infection occurred within 30 days and ataxia was present by 60 days. The assessment of ataxia was not as sensitive a parameter to detect S. neurona infection in the CNS as was the presence of cerebrospinal fluid antibodies and other signs of acute infection. The best indicator of EPM in the horse was gait analysis, cerebrospinal fluid antibodies and detection of early signs of infection. Because the animals were not immunocompromised, it was important to evaluate the horses for more than one 30-day observation period, in case the infection resolved. The reduction in cerebrospinal fluid antibodies may indicate that the immune system has effectively eliminated the parasite. However, in all cases, the CSF antibodies declined, and signs progressively worsened. If the parasite was eliminated, disease manifested by lameness, and ataxia may be a consequence of inflammation induced by parasite elimination. It is also possible that the parasite has an effect on the immune system to down-regulate immune responses. Therefore, the presence of the parasite would direct parasite-specific immunosuppression in the CNS, and signs of disease are a consequence of presence of the parasite in vital cells. It is difficult to detect the parasite after death in natural cases of EPM, indicating that the parasite has been eliminated. It remains to be determined at what stage S. neurona can be recovered from the CNS during experimental infections and if inflammation rather than active parasite play a role in ataxia. We have isolated parasites from the cerebrospinal fluid of challenged horses early in infections while there was a sustained parasitemia (day 7) and from the CNS tissues at 60 days after challenge,17 but acute signs were still apparent in these animals. Based on our observations we define acute disease as the presence of acute signs and an AI greater than 0.7. We defined chronic EPM as a presence of Grade 2 ataxia or lameness that was present on 2 consecutive examinations 30 days apart, presence of IgG in the serum with a titer of more than 100 or a twofold increase in titer.
References
1. Cusick PK, Sells DM, Hamilton DP, Hardenbrook HJ: Toxoplasmosis in two horses. J Am Vet Med Assoc 164:77-80, 1974.
2. Beech J, Dodd DC: Toxoplasma-like encephalomyelitis in the horse. Vet Pathol 11:87-96, 1974.
3. Dubey JP, Davis GW, Koestner A: Equine encephalomyelitis due to a protozoan parasite resembling Toxoplasma gondiil. J Am Vet Med Assoc 165:249-255, 1974.
4. Davis SW, Daft BM, Dubey JP: Sarcocystis neurona cultured in vitro from a horse with equine protozoal myelitis. Equine Vet J 23:315-317, 1991.
5. Dubey JP, Davis SW, Speer CA, Bloggs J: Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiological agent of equine protozoal myeloencephalitis. J Parasitol 77:212-218, 1991.
6. Cheadle MA, Yowell CA, Sellon DC, et al: The striped skunk (Mephitis mephitis) is an intermediate host for Sarcocystis neurona. Int J Parasitol 31:843-849, 2001.
7. Cheadle MA, Ginn PE, Lindsay DS, Greiner EC: Neurologic disease in gamma-interferon gene knockout mice caused by Sarcocystis neurona sporocysts collected from opossums fed armadillo muscle. Vet Parasitol 103:65-69, 2002.
8. Cutler TJ, MacKay RJ, Ginn PE, et al: Immunoconversion against Sarcocystis neurona in normal and dexamethasone-treated horses challenged with S. neurona sporocysts. Vet Parasitol 95:197-210, 2001.
9. Fenger CK, Granstrom DE, Gajadhar AA, et al: Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp. Sporocysts from the opossum (Didelphis virginiana). Vet Parasitol 68:199-213, 1977.
10. O'Donoghue PJ, Ford GE: The asexual pre-cyst development of Sacocystis tenella in experimentally infected specific-pathogen-free lambs. Int J Parasitol 14:345-355, 1984.
11. Johnson AJ, Hildebrandt PK, Fayer R: Experimentally induced Sarcocystis infection in calves. Pathol Am J Vet Res 36:995-999, 1975.
12. Fayer R, Leek RJ: Sarcocystis transmitted by blood transfusion. J Parasitol 65:890-893, 1979.
13. Fayer R: Multiplication of Sarcocystis bovicanis in the bovine bloodstream. J Parasitol 65:980-982, 1979.
14. Dubey JP: Parasitemia and early tissue localization of Sarcocystis neurona in interferon gamma gene knockout mice fed sporocysts. J Parasitol 87:1476-1479, 2001.
15. Rosypal AC, Lindsay DS, Duncan R, et al: Mice lacking the gene for inducible or endothelial nitric oxide are resistant to sporocyst induced Sarcocystis neurona infections. Vet Parasitol 103:315-321, 2002.
16. Dubey JP, Lindsay DS, Kwok OC, Shen SK: The gamma interferon knockout mouse model for Sarcocystis neurona: Comparison of infectivity of sporocysts and merozoites and routes of inoculation. J Parasitol 87:1171-1173, 2001.
17. Ellison SP, [AU: Please give up to 3 author names for ref 17.] et al: Molecular characterization of a major 29 kDa surface antigen of Sarcocystis neurona. Int J Parasitol 32:217-225, 2002.
18. Ellison SP, Kennedy T, Brown KK: Recombinant SG1 ELISA detects antibodies to Sarcocystis neurona in equine infections. Can J Parasitol Submitted for publication, 2003.
19. Ellison SP, Kennedy T, Greiner EC, Brown KK:
Experimental infection of horses with Sarcocystis neurona merozoites
as a model for equine protozoal myeloencephalitis. Can
J Parasitol Submitted for publication, 2003.
Figure 1. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 1 at 4 observation periods 30 days apart. This horse had serum
antibodies present at 30 days followed by an AI SAG1 index of 1.1 at
60 days and the presence of acute signs at 90 days.
During the observation period,
the horse was not ataxic.
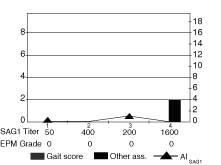
Figure 2. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 2 at 4 observation periods 30 days apart. This horse had serum
antibodies present at 60 days, an AI SAG1 index of 4.4 at 30 days and
the presence of acute signs at 30 days. This horse
was not ataxic but was lame and
the acute signs became progressively worse during the observation period.
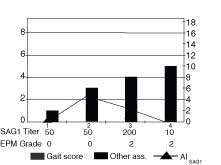
Figure 3. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 3 at 4 observation periods 30 days apart. This horse had serum
antibodies present at 60 days, an AI SAG1 index of 0.7 at 60 days and
the presence of acute signs at 30 days. During the
observation period, the horse was
not ataxic but was lame. the acute signs became progressively worse
during the observation period.
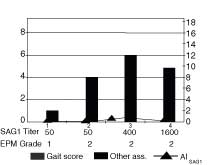
Figure 4. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 4 at 4 observation periods 30 days apart. This horse had serum
antibodies present at 30 days, an AI SAG1 index of 7.01 at 60 days and
the presence of acute signs that included lameness at 30 days. Ataxia,
Grade 2 in multiple limbs, was noted at 60 days.
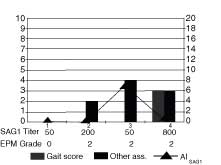
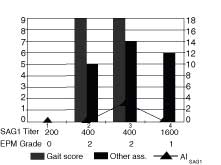
Figure 5. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 5 at 4 observation periods 30 days apart. This horse had serum
antibodies present at 60 days, an AI SAG1 index of 17.2 at 30 days and
the presence of acute signs that included lameness at 30 days. Ataxia,
Grade 2 in multiple limbs was noted at 60 days and became progressively
worse for the duration of the study.
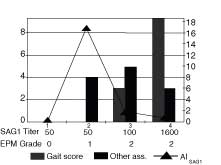
Figure 6. The AISAG1 (line), gait score and acute
signs (bars), serum titer and and EPM grade (table) are graphed for
Horse 6 at 4 observation periods 30 days apart. This horse had serum
antibodies present at selection and had a four fold increase in titer
by the end of the observation period. An AI SAG1 index of 3.3 at 60
days and the presence of acute signs that included lameness at 30 days
was seen. Ataxia, Grade 2 in multiple limbs, was noted at 30 days with
improvement in the ataxia but not the lameness by
the end of the observation period.
Horse 1 parked stance loose gait,
stumbles often
Horse 2
abnormal rigid drops
feed mentation cranial nerve lame
tail carriage change deficits left rear
Horse 3 drops feed mentation drools parked generalized lame
change stance weakness
Horse 4 drops feed drools cranial stumbles, generalized
nerve falls to weakness
deficits knees
Horse 5
drops feed abnormal generalized muscle lame stumbles
mentation weakness atrophy
often
Horse 6 drops feed facial paresis general severe lame muscle
weakness depression both rear atrophy
Figure 7. The early signs in six horses observed
by non-blinded observers are tabulated. The
initial signs were dropping feed and abnormal tongue tone followed by drooling and mentation
changes. Facial paresis was common. A generalized weakness, stumbling often falling to
knees, and lameness were seen. Muscle atrophy was observed in two horses. These
observations were not used to obtain the "other signs" data because blinded observers were
only present at formal neurological
examinations.
ISSN# 1542-2666