|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
Lactoferrin to Prevent
Experimental Escherichia coli Diarrhea in Weaned Pigs
L. Sarelli, DVM†
M. Heinonen, DVM, PhD‡
T. Johansson, Microbiologist PhD*
K. Heinonen, DVM, PhD§
H. Saloniemi, Professor†
†Faculty of Veterinary Medicine, Department
of Clinical Science,
‡Faculty of Veterinary
Medicine, Department of Clinical Veterinary Science, Saari Unit, Saarentaus,
Finland
*National Veterinary and Food Research
Institute, Department of Bacteriology, Helsinki, Finland
§Vetcare Oy, Finland
KEY WORDS: growth, hemoglobin, lactoferrin, pig, post-weaning diarrhea
ABSTRACT
Post-weaning diarrhea (PWD) is the major cause of economic loss in pig production, and new approaches for control of this condition are needed. In the present study, clinical effectiveness of orally administered bovine lactoferrin on experimental Escherichia coli (E. coli) diarrhea in weaned pigs was studied. Effect of Lactoferrin on blood hemoglobin concentration and weight gain of pigs was also monitored, and hemolytic fecal E. coli counts were performed. For the experiment, 36 pigs were assigned at random to 6 groups (n = 6). All pigs were weaned at the age of 28 to 34 days and challenged with E. coli one day after weaning. Lactoferrin (0.25–1.0 g/day) was given to 4 groups (A, B, C, and F) for 7 days before and 8 days after the challenge. Group D was a challenged control group and E and F were unchallenged control groups. The bacteriostatic effect of lactoferrin against the challenge strain in vitro was confirmed with turbidometry. Lactoferrin administration to the pigs, in different dosage levels, had no statistically significant effect on occurrence of diarrhea, hemolytic E. coli count in the feces, weight gain, or hemoglobin concentration in treated groups compared with the control groups. Lactoferrin, given twice daily, may have no protective effect against experimental E. coli diarrhea induced by massive bacterial inoculation. However, lactoferrin inhibited growth of the challenge strain in vitro, and the inhibition depended on the concentration of lactoferrin and bacteria. Further research is needed to show the antibacterial effect of lactoferrin in vivo.
INTRODUCTION
Post-weaning diarrhea (PWD) is the most significant disease problem in pig production during the first 2 weeks after weaning. Enterotoxigenic Escherichia coli is strongly associated with the pathogenesis of PWD.1-3
However, the etiology is considered to be multifactorial because environmental, nutritional, and immunologic factors are also involved.2,4
Lactoferrin, an iron-binding glycoprotein, is found in the colostrum, milk, and other secretions of many mammalian species. Lactoferrin is suggested to have several physiologic functions, including regulation of iron absorption, and it has an important role in host defence, including broad-spectrum antibacterial effects.5,6 Lactoferrin is bacteriostatic because it sequesters environmental iron, an essential growth factor of bacteria.7 In addition, lactoferrin can directly damage the outer membrane of gram-negative bacteria by releasing lipopolysaccharides (LPS) from the membrane,8,9 and it neutralizes the detrimental effects of LPS.9,10
Dionysius et al.11 studied in vitro 19 enterotoxigenic E. coli strains isolated from piglets with neonatal diarrhea or PWD, for their sensitivity to inhibition by lactoferrin. Lactoferrin at a concentration of 0.2 mg/mL inhibited 10 strains, and the remaining 9 strains were less sensitive but were inhibited by higher concentrations. In in vivo studies, orally administered lactoferrin suppressed Enterobacteriaceae and Clostridium species proliferation in the gastrointestinal tract of mice.12,13 Still et al.14 tested the clinical efficacy of a preparation containing lactoferrin and lactoperoxidase system (LP-s) on experimentally induced E. coli diarrhea in calves. Mortality, occurrence of severe diarrhea, and duration of diarrhea were significantly lower and clinical status was better in calves treated with lactoferrin and LP-s than in the control group. Van Leeuwen et al.15 reported that a combination of lactoferrin and LP-s given orally decreased E. coli counts in the intestine and feces of calves and also reduced the severity of diarrhea. A recent report suggests that orally administered lactoferrin can protect neonatal rats from experimental, gut-related, systemic E. coli infection.16 Lee et al.17 reported that lactoferrin (per os) protects piglets against lethal shock induced by intravenously administered E. coli LPS (endotoxin). Piglets fed with lactoferrin had significantly lower mortality compared with piglets fed with bovine serum albumine (16.7% vs. 73% mortality).
Previously, low doses of antimicrobials in pig feeds were commonly used to control PWD. Restricted use of antimicrobials decreases selection pressure of microbes to resistance.18 However, antimicrobials are still frequently used in pig production, and novel therapies are required to aid the reduction of levels of antimicrobial resistance.
The aim of the present study was to investigate the clinical effectiveness of bovine lactoferrin in preventing experimental PWD in pigs. Other aims were to monitor the effect of lactoferrin on fecal E. coli count, blood haemoglobin concentration, and weight gain. The bacteriostatic effect of lactoferrin on the challenge strain was confirmed in vitro.
MATERIALS AND METHODS
Animals and Management
Three pregnant Yorkshire gilts and one sow were obtained from a conventional herd free from major infectious diseases. The animals were vaccinated against parvovirus and Erysipelothrix rhusiopathiae (Nordpremum Plus, Pharmacia & Upjohn Animal Health AB, Kaarina, Finland) according to the manufacturer’s instructions.
Piglets were raised with their own litter in the same pen until weaning. Three or four days post-partum, the piglets were injected subcutaneously with 200 mg Fe2+ (Pigfer-Se vet, Orion Pharma, Espoo, Finland) and the males were castrated. The same dry piglet feed (Porsaan-herkku, Rehuraisio, Raisio, Finland) was used ad libitum from the age of 1 week until the end of the experiment. The piglets were weaned at the age of 28 to 34 days. Heat lamps were removed from the pens after weaning to induce mild cold stress to piglets.
Lactoferrin
Lactoferrin was purified from cheese whey or concentrated cheese whey by an expanded bed adsorption chromatography method described by Isomäki.19 Iron content of lactoferrin was approximately 8% to 15%.19 Lactoferrin was stored at 4°C until the day before administration, when the lactoferrin dose was diluted with 3 mL of sterile water.
Challenge Strain
Escherichia coli O147 isolated from the intestine of a pig suffering from PWD (EELA 475) was used as a challenge strain. The strain had F4 fimbria (F4+) and it produced heat labile (LT+) and heat stabile (ST2+) enterotoxines. The strain was resistant to ampicillin, streptomycin, sulphatrimethoprim, tetracycline, and cloramphenicol, and displayed wide hemolytic zones around the colonies on sheep blood agar.
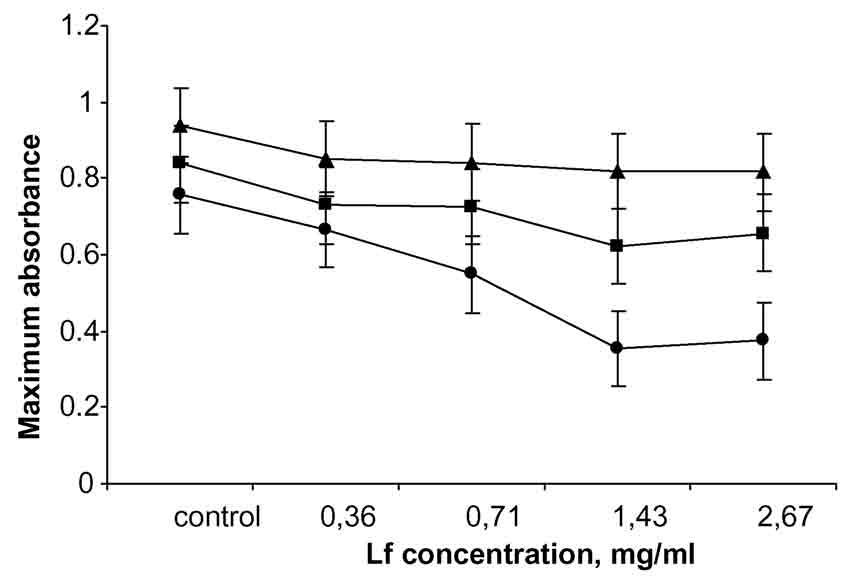
In vitro study: The antibacterial effect of lactoferrin on the E. coli challenge strain was studied in vitro using the turbidometric method.20 Iso Sensitest-Broth (ISB CM473, Oxoid Ltd., Basingstoke, Hampshire, England) was used in a 50% dilution as a growth medium. Concentrations of lactoferrin tested in the study were 0.36, 0.71, 1.43, and 2.67 mg/mL, and a 0.9% NaCl solution was used as a control (n = 5). Bacterial inoculum doses were 104, 106, and 108 colony forming units/mL (CFU/mL). Maximum absorbency (the highest absorbance value measured during the 24-hour incubation period at 37˚C) was used as a variable describing the bacterial growth.
Experimental Design
The piglets were randomly assigned to 6 groups (A, B, C, D, E, and F, Table 1) with equal numbers of littermates and both sexes per group of 6 piglets. Lactoferrin (0.25–1.0 g/d) was given to the piglets of 4 groups (A, B, C, and F) orally twice a day for 15 days: 7 days (days –7 to –1) before and 8 days (days 0 to 7) after E. coli challenge (Table 1). The animals in group D (challenged control) and E (unchallenged control) were not treated with lactoferrin.
One day after weaning, 1.1 x 1010 CFU of the challenge strain as an overnight culture (18 h at 37˚C) in brain heart infusion broth (20 mL) (BHI; Cat. No. 0037; Difco Laboratories, Detroit, MI, USA) were given with an orogastric tube to the piglets of groups A, B, C, and D. Sterile BHI (20 mL) was given to the unchallenged control piglets in groups E and F, which were housed in a separate room from the challenged pigs.
Clinical Evaluation
A clinical examination was made just before E. coli challenge, every 6 hours after challenge at day 0, and thereafter twice a day until the end of the experiment (day 7) by a single veterinarian. Rectal temperature was measured, degree of dehydration, anorexia, and depression were evaluated, and consistency of feces was scored (0 = normal dry faeces, 1 = moist feces, 2 = diarrhea, 3 = watery diarrhea). Body weight was recorded daily during the experiment and once a week until the pigs were 10 weeks old. The 2 pigs that died were examined post mortem.
Fecal specimens were obtained with rectal swabs before challenge (day –1) and once a day for the next 4 days after challenge and at day 6. The specimens were stored at –70˚C until analyzed for hemolytic E. coli counts. Blood samples for hemoglobin determination were taken once before lactoferrin dosing (day –13), at the end of the experiment (day 8), and 2 weeks later (day 23). The samples were collected from the vena cephalica accessoria with EDTA vacutainer tubes. Hemoglobin was measured with an automated hematology analyzer (CELL-DYN 3500, Abbott Laboratories, IL).
Enumeration of E. coli
Hemolytic E. coli counts in the fecal samples were performed within 3 months after the end of the experiment. Fecal samples (6–90 mg) were suspended in a diluent containing 0.1% peptone in 0.85% saline in a ratio of 1:99. The initial suspensions and their tenfold serial dilutions (0.1 mL) were cultured in duplicate on sheep blood agar plates (Trypticase soy agar, TSA; BBL Cat. No. 11043; Becton Dickinson and Company, Cockeysville, MD, supplemented with 5% sheep blood, sodium citrate used as anticoagulant) containing streptomycin (Sigma S-9137; Sigma Chemical, St. Louis, MO) 20 mg/L and oxytetracycline (Sigma O-5875) 30 mg/L. Typical hemolytic colonies were counted after incubation for 24 hours at 37˚C. Sheep blood and antibiotics allowed specific enumeration of challenge E. coli without further confirmation.
Statistical Analysis
The effect of different lactoferrin concentrations, bacterial inoculum dosages, and their interaction on maximum absorbance was tested using analysis of variance (ANOVA). Repeated measures analysis of variance with measurement day as within factor and treatment group as a between factor was used to test the effect of lactoferrin on weight gain, blood hemoglobin concentration, and E. coli count in feces during the trial. Greenhouse-Geisser adjusted P values were used to evaluate the significance of within factors. Kruskal Wallis nonparametric ANOVA was used to test differences between treatment groups in diarrhea scores on each observation day. P values less than .05 were considered significant.
RESULTS
In in vitro study, lactoferrin had a significant effect on maximum absorbance in different concentrations (P < .001) compared with the control, but the effect of lactoferrin in concentrations of 1.43 and 2.67 mg/mL did not differ significantly from each other. In addition, a significant interaction (P < .001) was observed between bacterial inoculum dosage and lactoferrin. Increasing lactoferrin concentration inhibited bacterial growth more with decreasing bacterial inoculum dosage (Fig. 1).
All 36 pigs were clinically normal during the first week of the experiment (days –7 to 0) and at the time of the bacterial challenge. Diarrhea (score 2 to 3) occurred in 17 of 24 challenged pigs and transient diarrhea in 3 of 12 unchallenged control pigs during the 8-day observation period after the challenge (Table 1). The difference in occurrence of diarrhea between challenged and unchallenged groups was statistically significant at days 0, 1, and 3 (P < .05). The same pigs may have suffered transient diarrhea several times during the period. No severe depression, dehydration, or anorexia was observed in any group, and moderate signs were observed only in group A.
The difference in occurrence of diarrhea among the challenged groups with different lactoferrin dosages (0-1.0 g/d) was not statistically significant. In group A (lactoferrin 0.25 g) 5 of 6 pigs had diarrhea (score, 2 to 3) during 8 days after challenge lasting 12 hours to 4 days. One pig from group A died at day 3 and another at day 10 after challenge, both from a perforated gastric ulcer. The third pig in group A was given enrofloxacin 5 mg/kg subcutaneously (Baytril vet 100 mg/mL, Bayer AG, Germany) from day 4 to 6 for treatment of severe diarrhea. The treated pig was euthanized at the end of the experiment because of retarded growth. In each of the groups B (lactoferrin 0.5 g), C (lactoferrin 1.0 g), and D (challenged control) 4 of 6 pigs experienced diarrhea (score, 2 to 3). Duration of diarrhea was from 12 hours to 2 days in group B, and 12 hours to 4 days in groups C and D. In the unchallenged control group E, 2 of 6 (at days 5 and 6) and in F, 1 of 6 pigs (at day 3) experienced diarrhea (score 2) lasting for 12 hours.
Before challenge, hemolytic E. coli counts in feces were less than 1,000 CFU/g in all groups, and they remained low in the unchallenged groups (E and F), except in the 3 pigs with transient diarrhea. Mean bacterial counts of challenged groups are shown in Figure 2, and the number of pigs per group shedding over 108 CFU/g hemolytic E. coli in feces are presented in Table 1. No statistically significant differences were seen in bacterial counts between challenged groups.
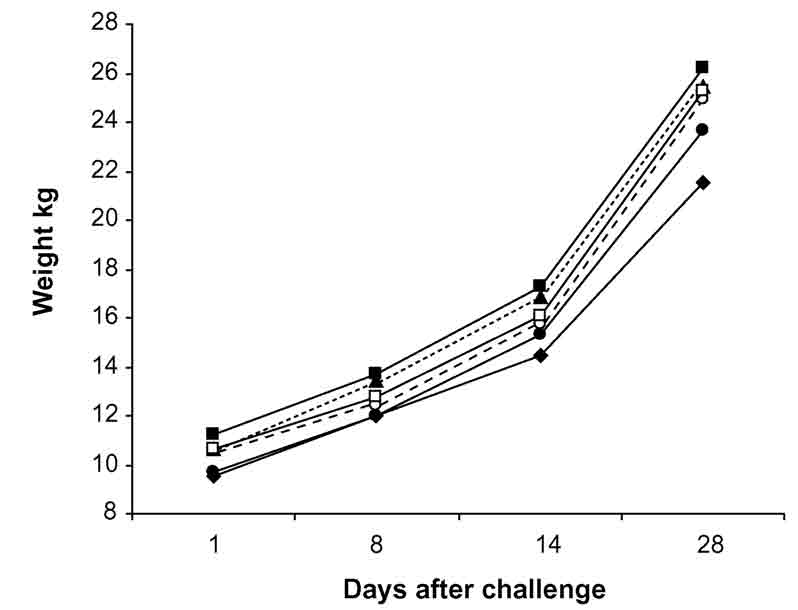
Mean weight gains during days 1 to 28 after challenge were not significantly influenced by lactoferrin treatment or bacterial challenge (Fig. 3). Mean daily weight gain of all groups during the periods of days 1 to 8 and 1to 28 after challenge was 328 g (standard error of mean [SE], 14.5) and 529 g (SE, 13.5), respectively. Lactoferrin treatment had no significant effect on blood hemoglobin concentration tested on days 8 and 23 after challenge (Fig. 4). Before the beginning of the trial, the mean hemoglobin concentration of all the groups was 110 g/L (SE, 2.0), and 102 g/L (SE, 2.0) and 111 g/L (SE, 1.0) on days 8 and 23 after challenge, respectively.
DISCUSSION
Lactoferrin, given orally in different dosages before and after E. coli challenge, did not significantly reduce the occurrence of experimentally induced diarrhea or hemolytic E. coli counts in feces. The weight gains or hemoglobin concentrations in the various treatment groups were not significantly affected. The bacteriostatic effect of lactoferrin on the challenge strain was confirmed in vitro with turbidometry. The effect was dose dependent and also dependent on bacterial inoculum dosage because the bacteriostatic effect was stronger when the inoculum dosage was smaller.
Severe systemic symptoms (dehydration, depression) were not seen in any groups, although some pigs suffered moderate to severe (watery) diarrhea. This may be due to the fact that the management of the pigs was very good, except for the mild cold stress to which the pigs were subjected. Other studies have reported that the typical signs of PWD observed in field cases (chronic enteritis lasting several days, reduced growth), may be difficult to induce experimentally.4,21 Moderate systemic symptoms were seen only in group A, but 2 pigs in this group also experienced gastric ulcer.
The exact role of lactoferrin in iron absorption is not fully understood. Iron is a critical nutrient of growing pigs, lactoferrin is the main iron-binding protein in sow milk, and piglets have specific receptors for lactoferrin in the small intestine.22 Some studies suggest that lactoferrin may facilitate iron absorption in infants and laboratory animals.5,23 Other studies have failed to demonstrate an improvement in iron absorption, but the iron bound in lactoferrin has been found to be bioavailable in the intestine.24,25 Our results would support the theory that lactoferrin has no effect on iron absorption in pig intestine, because no significant differences were seen in blood hemoglobin concentrations between the groups.
In conclusion, lactoferrin has no protective effect
on experimental E. coli diarrhea in weaned pigs. This result is in contrast
to other reports for other animal species. Still et al.14 and van Leeuwen
et al.15 reported that duration and severity of diarrhea in calves were
reduced in lactoferrin-treated groups compared with control groups.
They used a combination of LP-s and lactoferrin, and the preparation
was administered to calves in milk. Other studies have also shown that
lactoferrin alone has an antibacterial effect in systemic and intestinal
infections of many animal species.12,13,16,17 In our study, lactoferrin
was administered only twice a day, and it may be necessary for lactoferrin
to be continuously available in the intestine to have antibacterial
action. This could be achieved if lactoferrin were administered in feed.
The antibacterial effect also depends on the dosages of lactoferrin
and bacteria as was seen in vitro. In this study, a massive bacterial
challenge was inoculated via orogastric tube, whereas in field cases
of PWD such massive bacterial challenge does not occur at once. It may
be that under these circumstances, the antibacterial effect of lactoferrin
is not exceeded. Further field research is needed to test this hypothesis.
ACKNOWLEDGMENTS
We thank Liisa Myllykoski at the University of Oulu, Finland, for providing lactoferrin, statistician Arto Ketola for statistical analyses, Lasse Nuotio and Laura Hänninen for advice, Ilkka Linna, Mia Dufva, and Taina Taival for assistance with animals, and Ilkka Saastamoinen, Minna Pietikäinen, Kaija Pajunen, and Susan Sundqvist for technical assistance.
REFERENCES
1. Frydendahl K: Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhea and edema disease in pigs and a comparison of diagnostic approaches. Vet Microbiol 85:169–182, 2002.
2. Hampson DJ: Postweaning Escherichia coli diarrhoea in pigs. In Gyles CL: Escherichia coli in domestic animals and humans. pp. 171–191. Wallingford: CAB International, 1994.
3. Imberechts H, Bertschinger HU, Stamm M, et al: Prevalence of F107 fimbriae on Escherichia coli isolated from pigs with oedema disease or postweaning diarrhoea. Vet Microbiol 40:219–230, 1994.
4. Madec F, Bridoux N, Bounaix S, et al: Experimental models of porcine post-weaning colibacillosis and their relationship to post-weaning diarrhoea and digestive disorders as encountered in the field. Vet Microbiol 72:295–310, 2000.
5. Chierici R, Sawatzki G, Tamisari L, et al: Supplementation of adapted formula with bovine lactoferrin. 2: Effects of serum iron, ferritin and zinc levels. Acta Paedietrica 81:475–479, 1992.
6. Lönnerdal B, Iyer S: Lactoferrin: Molecular structure and biological function. Ann Rev Nutr 5:93–110, 1995.
7. Ellison III RT: The effects of lactoferrin on gram-negative bacteria. In Hutchens et al., ed: Lactoferrin: Structure and Function. pp 71–90. New York: Plenum Press, 1994.
8. Ellison III RT, Giehl TJ, LaForce FM: Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferring. Infect Immun 56:2774–2781, 1988.
9. Appelmelk BJ, An Y-Q, Geerts M, et al: Lactoferrin is a lipid A-binding protein. Infect Immun 62:2628-2632, 1994.
10. Zhang G-H, Mann D, Tsai C-M: Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect Immun 67:1353–1358, 1999.
11. Dionysius DA, Grieve PA, Milne JM: Forms of lactoferrin: their antibacterial effect on enterotoxigenic Escherichia coli. J Dairy Sci 76:2597–2606, 1993.
12. Teraguchi S, Ozawa K, Yasuda S, et al: The bacteriostatic effects of orally administered bovine lactoferrin on intestinal Enterobacteriaceae of SPF mice fed bovine milk. Biosci Biotechnol Biochem 58:482–487, 1994.
13. Teraguchi S, Shin K, Ozawa K, et al: Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl Environ Microbiol 61:501–506, 1995.
14. Still J, Delahaut P, Coppe P, et al: Treatment of induced enterotoxigenic colibacillosis (scours) in calves by the lactoperoxidase system and lactoferrin. Annales Recherche Vétérinaires 21:143–152, 1990.
15. van Leeuwen P, Oosting SJ, Mouwen JMVM, Verstegen MWA: Effects of a lactoperoxidase system and lactoferrin, added to a milk replacer diet, on severity of diarrhoea, intestinal morphology and microbiology of digesta and faeces in young calves. J Animal Physiol Animal Nutr 83:15–23, 2000.
16. Edde L, Hipolito RB, Hwang FFY, et al: Lactoferrin protects neonatal rats from gut-related systemic infection. Gastrointest Liver Physiol 281:1140–1150, 2001.
17. Lee WJ, Farmer JL, Hilty M, Kim YB: The protective effect of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect Immun 66:1421–1426, 1998.
18. Gold HS, Moellering RC: Antimicrobial-drug resistance. N Engl J Med 335:1445–1453, 1996.
19. Isomäki R: Separation of whey antimicrobial proteins and development of bovine lactoferrin immunoassays. Licenciate thesis. University of Oulu, Finland, 1999.
20. Kutila TM, Pyörälä SHK, Saloniemi HS, Kaartinen LA: Antibacterial effect of bovine lactoferrin against udder pathogens. [AU: Please give journal name and update, if available, for ref 20.] In press, 2002.
21. Melin L, Katouli M, Lindberg Å, et al: Weaning of piglets: Effects of an exposure to a pathogenic strain of Escherichia coli. J Vet Med B 47:663–675, 2000.
22. Gíslason J, Iyer S, Douglas GC, et al: Binding of porcine milk lactoferrin to piglet intestinal lactoferrin receptor. In Hutchens TW, et al: Lactoferrin: Structure and Function. pp. 239–244. New York: Plenum Press, 1994.
23. Kawakami H, Hiratsuka M, Dosako S: Effects of iron-saturated lactoferrin on iron absorption. Agricultural Biol Chem 52:903–908, 1988.
24. Davidsson
L, Kastenmayer P, Yuen M, et al: Influence of lactoferrin on iron absorption
from human milk in infants. Pediatr Res 35:117–124, 1994.
25. Fransson G-B, Keen CL, Lönnerdal B: Supplementation
of milk with iron bound to lactoferrin using weanling mice: I. Effects
on haematology and tissue iron. J Pediatr Gastroenterol Nutr 2:693–700,
1983.
| Group | Lf dose g/day | Bacterial challenge | Days after challenge | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
|
Number
of pigs with diarrhoea and (shedding)
|
||||||||||
| A | 0.25 | Yes | 4 | 2(3) | 2(2) | 2(2) | 3(2) | 0 | 0(0) | 1 |
| B | 0.5 | Yes | 2 | 1(1) | 0(0) | 1(3) | 1(3) | 1 | 0(0) | 0 |
| C | 1.0 | Yes | 0 | 2(1) | 1(1) | 4(4) | 1(3) | 1 | 0(0) | 0 |
| D | No | Yes | 4 | 1(1) | 1(0) | 3(0) | 0(1) | 0 | 0(0) | 0 |
| E | No | No | 0 | 0(0) | 0(nd) | 0(nd) | 0(0) | 1 | 1(0) | 0 |
| F | 0.5 | No | 0 | 0(0) | 0(nd) | 1(nd) | 0(0) | 0 | 0(0) | 0 |
N=6, except in group A n=5 from 4 to7
Nd=not
done
Figure 1. The effect of lactoferrin concentrations
and different inoculum dosages of E. coli (•104, n106,
and s108
CFU/mL) on maximum absorbance measured with turbidometry (n = 5). Lactoferrin
concentration and bacterial inoculum dosage had a significant interaction
effect on maximum absorbance (P < .001).
Figure
2. The mean number (CFU/g) of hemolytic E. coli in feces of pigs
weaned at day –1 and orally challenged with E. coli at day 0. Three groups were orally administered with lactoferrin
(u A, 0.25 g/d, n = 5; n B, 0.5 g/d, n =
6; s
C, 1.0 g/d, n = 6; • D, 0 g/d, n = 6)
for 7 days before and 8 days after the challenge. P > .05.
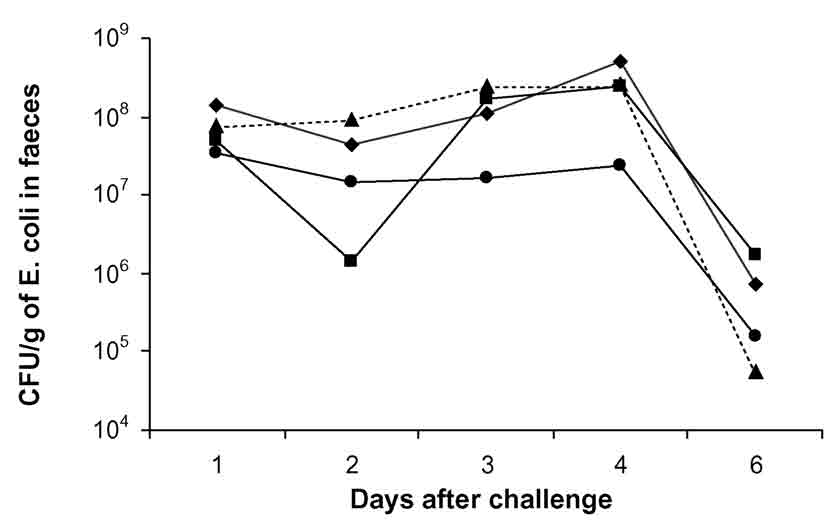
Figure 3. The mean weight gain of groups of pigs
after weaning at day –1, E. coli challenge at day 0, and oral lactoferrin
treatment 7 days before and 8 days after the challenge (P >
.05). Groups A, B, C, and D were challenged with E. coli,
and groups E and F served as unchallenged controls. The groups were
treated with lactoferrin: u A,
0.25 g/d;
n
B,
0.5 g/d; s
C,
1.0 g/d; •
D,
challenged control group 0 g/d; • E,
unchallenged control group 0 g/d; n F,
0.5 g/d (n = 6, except in group A; n = 3 at days 8, 14, and 28.
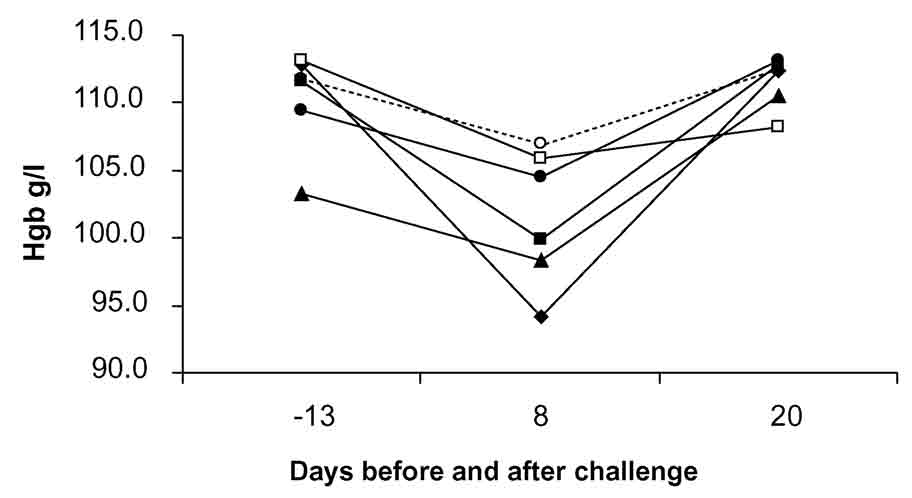
Figure 4. The mean blood hemoglobin (Hgb) concentration
of pigs after weaning at day –1, E. coli challenge
at day 0 and oral lactoferrin treatment 7 days before and 8 days after
the challenge (P
> .05). Groups A, B, C, and D were challenged with E. coli,
and groups E and F served as unchallenged controls. The groups were
treated with lactoferrin: u A
0.25 g/d; n
B,
0.5 g/day; s
C,
1.0; g/day,
• D
challenged control group 0 g/day, • E
unchallenged control group 0 g/day, n F
0,5 g/day. n = 6, except in group A; n = 3 at days 8 and 20.
ISSN# 1542-2666