|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
The
Effects of Oral Glutamine Supplementation on Plasma Glutamine Concentrations
and PGE2 Concentrations in Dogs Experiencing Radiation-Induced
Mucositis
Susan E. Lana, DVM, MS†
Rodney A. Hansen, PhD†
Lauren Kloer†
Susan M. LaRue, DVM, PhD‡
Annette M. Bachand, PhD‡
Gregory K. Ogilvie, DVM†
†The Animal Cancer Center, Department of Clinical Sciences, and
‡The Department of Environmental and Radiological
Health Sciences, College of Veterinary Medicine and Biomedical Sciences,
Colorado State University, Fort Collins, Colorado
Sponsored in part by the College Research Council (Miki
Society), College of Veterinary Medicine and Biomedical Sciences, Colorado
State University.
KEY WORDS: prostaglandin E2, mucosal tissue, dogs, glutamine, radiation-induced mucositis
Abstract
Objective: The purpose of this study was to determine the effects of oral glutamine supplementation on plasma glutamine concentrations and prostaglandin E2 (PGE2) concentrations in the mucosal tissue of dogs experiencing radiation-induced mucositis.
Sample Population: Canine patients with spontaneously occurring head and neck malignancies undergoing curative intent radiation therapy
Procedure: Twenty-one patients received radiation on a Monday through Friday schedule for a median total dose of 54 Gy. Each dog was concurrently treated with oral L-glutamine suspension at a dose of 4mg/m2 starting on the first day of radiation therapy and continuing for 5.5 weeks. Plasma samples were collected at the following time points: pretreatment, week 1, end of treatment, and 1 month after treatment. Mucosal tissue biopsies were taken at the pretreatment and end of treatment time points. Oral mucositis scores and weights were also recorded. Six nonsupplemented dogs with the same treatment course had similar samples taken and were used for comparison.
Results: Oral mucositis scores indicating radiation-induced
tissue damage increased as expected in all dogs. Mean tissue PGE2 levels were higher at the end of radiation treatment
than before treatment in both groups; however, the increase was significantly
higher in the control group (P = .006). No
differences were seen over time or between groups in any other parameter
measured (plasma glutamine, plasma bicyclo PGE2, weight).
Conclusions: Oral glutamine supplementation was well tolerated. Although a possible biologic effect was seen, the clinical usefulness of this supplement in the modulation of radiation induced mucositis requires further study in a controlled, randomized fashion.
INTRODUCTION
Glutamine is the most abundant amino acid in the blood and in the free amino acid pool.1 It has multiple functions, being a regulator of protein synthesis, the most important substrate of renal ammoniagenesis, and an essential precursor for nucleic acid biosynthesis in all cells.2 Glutamine is the key fuel for rapidly dividing cells, such as enterocytes, renal tubular cells, lymphocytes, and malignant cells.1-3 Several studies report that glutamine has recently been recognized as an important substrate for the intestinal mucosa to maintain growth and function, particularly during times of mucosal damage.4,5 During critical illness, the balance of glutamine metabolism switches to favor enhanced use, leading to a state of total body glutamine depletion and a catabolic state.2,3 Concentrations of glutamine are found to be decreased during starvation, metabolic acidosis, postoperative stress, and advanced malignant states.3 In septic patients, intracellular glutamine stores in skeletal muscle may fall by 75%, and the extent of this decrease correlates with survival.3 Because of this, glutamine is now thought of as a conditionally essential amino acid during times of illness.6
Administration of glutamine to healthy and diseased human subjects leads to an increase in plasma and tissue glutamine levels, and has not been associated with significant side effects.6,7 Several studies have shown that supplementation with glutamine has been shown to reduce systemic illness or tissue toxicity due to radiation and chemotherapy. In rodent chemotherapy-induced enterocolitis models, glutamine supplementation improved nutritional status and decreased the amount of intestinal injury seen histologically. Decreased bacteremia and sepsis were also noted, and researchers concluded that glutamine supplementation resulted in improved survival after methotrexate administration.4,8 In another study, rats that received glutamine before abdominal radiation were protected from gut injury and had significantly increased jejunal villous number, villous height, and intact mucosa when compared with those who did not receive glutamine.5 Oral glutamine supplementation was also evaluated in a randomized, placebo-controlled trial in human patients experiencing chemotherapy-induced stomatitis. The glutamine supplemented group had a significant reduction in the severity and duration of the pain experienced compared with the placebo group.9
Animals undergoing radiation to the head and neck can experience severe mucosal injury that can lead to a decrease in food intake, increased pain, and a decrease in quality of life.10 Several amino acids, including glutamine, have been shown to be decreased in tumor-bearing dogs compared with in normal dogs.11 A recent study looking at acute effects of radiation in a group of 12 dogs showed a decrease in plasma glutamine levels.(Anderson et al., personnel communication, 1997) The levels were lowest when oral mucositis was considered most severe. Based on the above findings, it is reasonable to assume that supplementing radiation patients with an oral glutamine suspension may help reduce these effects.
Another mechanism by which glutamine may help decrease mucous membrane injury induced by radiation is by altering the inflammatory response. Glutamine has been shown to be a regulator of glutathione, a ubiquitous antioxidant.12 Glutathione is an antagonist to prostaglandin E2 (PGE2) production, which is a strong inflammatory mediator. Klimberg et al.13 used a rat breast cancer model to show that glutamine-supplemented rats with mammary tumors had greater glutamine and glutathione concentrations, and decreased PGE2 production, than rats that received no glutamine. In another study, PGE2 levels from tissues obtained by serial mucosal biopsies from dogs experiencing acute radiation effects increased with increasing inflammation (Anderson et al., personnel communication, 1997) The highest concentrations were documented when radiation-induced mucositis was at its worst. It is possible that oral glutamine supplementation in canine patients experiencing radiation-induced mucositis may alter tissue or plasma PGE2 concentrations.
The purpose of this study is to asses the effect of oral glutamine supplementation on plasma glutamine concentrations and tissue and plasma PGE2 concentrations, in canine patients experiencing radiation-induced mucositis. We hypothesize that oral glutamine supplementation will increase plasma glutamine concentrations and decrease plasma and tissue PGE2 concentrations.
Materials and Methods
Canine patients seen at the Animal Cancer Center at Colorado State University Veterinary Teaching Hospital that had been histologically diagnosed with spontaneously occurring malignancy of the head and neck that received curative-intent radiation therapy were eligible for the study. Informed client consent and University Animal Care and Use Committee approval were obtained before beginning the study. The treatment regimen for all patients was a minimum total radiation dose of 54 Gy, given in 18 daily fractions (3 Gy each) administered daily, Monday thru Friday. Computerized treatment planning was performed, and radiation was delivered using a 6-MV linear accelerator. This treatment schedule resulted in clinical mucositis associated with radiation therapy in all patients treated. L-glutamine solution was prepared as a suspension in Ora Sweet/Ora Plus (Paddock Laboratories Inc., Minneapolis, MN) suspending agent at a concentration of 500 mg/ml. L-glutamine was administered at a dose of 4 g/M2/d divided 3 times a day. The first daily dose was given as an oral “rinse” while the patient was anesthetized for radiation treatment, to maximize contact with the oral mucosa. The remaining 2 doses were administered orally, approximately 8 hours apart, with the patient awake. L-glutamine supplementation began on the first day of radiation therapy and continued until 2 weeks after therapy was completed (approximately 5.5 weeks).
Subjective assessment of the patients’ clinical status was measured daily by members of the clinical staff during radiation therapy treatment. Each patient was assessed using a “mucositis score” (Table 1) to quantitate the duration and severity of radiation-induced damage to the oral mucosa. It was adopted from the Veterinary Radiation Therapy Oncology Group (VRTOG) acute toxicity scoring system. Patients were monitored throughout radiation therapy using biochemical profiles and complete blood counts performed at the beginning, middle, and end of treatment.
Twelve mL of whole blood was obtained from each patient in sodium heparin tubes at the following time points: pretreatment, week 1 (mucositis not severe), end of treatment (mucositis usually severe), and 1 month after therapy (mucositis resolved). Samples were centrifuged, and the supernatant removed and frozen at –80˚C until assayed. Two hundred mL of plasma was obtained and mixed with 200 mL of sulfosalicylic acid (275 mmol/L), centrifuged, and the supernatant frozen at –80˚C until assayed for plasma glutamine concentrations. A mucosal punch biopsy (6 mm) was obtained immediately before and at the end of the radiation treatment protocol and flash-frozen in liquid nitrogen. The tissue was then stored at –80˚C until assayed. Plasma L-glutamine levels were measured using high-performance liquid chromatography (HPLC) as previously described.14,15 Briefly, the acid supernatant was thawed and centrifuged at 4˚C at 14,000 X g for 20 minutes, the supernatant was filtered through a 0.45-mm filter and to 100 mL of this supernatant was added 12.5 mL of 0.8 moles/L of lithium hydroxide and 12.5 mL of a solution containing 2 mmols/L of the internal standard, L-alpha-amino beta-guanidino propionic acid, and after mixing, 50 mL of this solution was placed on the column, using an automated amino acid analyzer (7300 Beckman Amino Acid analyzer, Beckman Instruments, Palo Alto, CA). The results are reported in nmols/mL plasma.
For tissue PGE2 determination, tissue samples were extracted using
an ethyl acetate method. Briefly, frozen tissue samples were minced,
placed in 4°C ethyl acetate and homogenized using a mechanical
homogenizer. Samples were centrifuged at 4˚C, 2000 X g and the
supernatant collected. The ethyl acetate was evaporated using a stream
of nitrogen gas. The residue was resuspended in EIA buffer (provided
by the kit manufacturer) and stored at –80°C until analysis
for PGE2 using a commercially
available enzyme immunosorbant assay (EIA) kit (Cayman Chemical Co,
Ann Arbor, MI).
PGE2 is rapidly converted to metabolites in vivo so that very little intact PGE2 is present in the blood or urine of human or veterinary patients. Therefore, measurement of a metabolite is a more accurate representation of actual PGE2 concentrations in those fluids.16 The Bicyclo PGE2 assay converts PGE2 metabolites into a stable derivative measured by enzyme EIA (Cayman Chemical Co.).17 Briefly, 500 uL plasma was mixed with 2 mL of ethanol, incubated, and centrifuged. The liquid phase was then decanted, and the ethanol was evaporated and mixed with 1 mL of EIA buffer. The sample was then passed through an activated 6 mL C-18 SPA cartridge (Supelco, Bellefonte, PA), eluted with 5 mL ethyl acetate with 1% methanol, and evaporated. Five hundred mL of EIA buffer was added, and the sample was vortexed. To derivitize the sample, 150 mL of carbonate buffer was added, the sample was incubated at 37˚C overnight, followed by 400 mL of phosphate buffer and 300 mL of EIA buffer. To the precoated 96-well plate, 50 mL of standard or sample, 50 mL of antibody and 50 mL of tracer were added to each well, and the plate was incubated. The plates were washed, 200 mL of Ellman’s reagent was added each well, and the plate was read at 405 nm.
For the control comparison, tissue and plasma samples from a group of similarly treated dogs (n = 6) that underwent the same radiation protocol for spontaneously occurring head and neck malignancies and did not receive glutamine supplementation were assayed using the previously mentioned methodologies (tissue PGE2 and glutamine). These patients were not intended to be a randomized control group and received treatment under a different study protocol several years earlier. (Anderson et al., personnel communication, 1997).
Statistical Analysis
Means and standard deviations were calculated for the outcome variables of interest among supplemented and control dogs using SAS version 8.02 (SAS Institute, Cary, NC). Concentrations of tissue PGE2, plasma bicyclo PGE2, and glutamine were analyzed using 1-way and 2-way analysis of variance (ANOVA) with repeated measures, in which supplementation was the between-group comparison and time was the within-group comparison, followed by pairwise comparisons via contrast statements. Assumptions were tested and found to be satisfied except in one case in which tests for normality indicated the need to log transform a variable. Significance was set at P < .05.
Results
Twenty one patients diagnosed with head and neck malignancy and that received curative-intent radiation therapy were included in the study. Histologic tumor types included 14 carcinomas and 7 sarcomas. Locations of the primary tumors are as follows: nasal cavity (n = 17), salivary gland (n = 1), maxilla (n = 2), and ear canal (n = 1). Radiation was delivered in daily fractions on a Monday through Friday schedule. Eighteen patients received a total radiation dose of 54 Gy given over 18 fractions. The 3 remaining patients had greater than 18 daily fractions (19fx, 20fx, 20fx) for total doses of 57, 54, and 57 Gy, respectively. All 17 patients with tumors in the nasal cavity also received cisplatin chemotherapy in the form of a biodegradable polymer formulation, which is used as a radiation sensitizer.16 All patients, regardless of tumor type or location, developed oral mucositis during the course of treatment. This was evident clinically and is reflected in increases in mucositis scores seen as treatment progressed (Fig. 1). Based on daily patient monitoring, periodic biochemical profiles, and complete blood counts, no adverse effects of glutamine administration were noted.
Mean tissue PGE2 values increased at the end of the radiation treatment period compared with the start of radiation in both supplemented and unsupplemented dogs, and the difference between the patterns of PGE2 increase in the supplemented and control groups was not statistically significant (P = .19). However, the increase at the end of radiation therapy was greater and statistically significant in the control group (P = .006) (Fig. 2). Plasma PGE2 values, measured as a bicyclo metabolite remained fairly constant over time in the supplemented dogs (Fig. 3). These values were not measured in the control dogs because appropriate samples were not available.
Mean plasma glutamine levels (reported in nanmoles per milliliter) in the supplemented dogs remained constant during the treatment and supplementation period, and dropped considerably at the 1-month post-treatment time point. In the control dogs, glutamine levels dropped at the end of treatment time point and then rebounded to pretreatment levels at 1 month after treatment (Fig. 4). None of the changes seen in glutamine values within groups were statistically significant. The difference between the patterns of plasma glutamine changes in the supplemented and control groups was also not statistically significant (p = .57). Body weights remained constant throughout the treatment period for both groups.
Discussion
Supplementation with oral glutamine solution was well tolerated, with no adverse effects seen in any patients in this study. This treatment, however, did not abrogate the clinically observable changes associated with radiation-induced mucositis. In a small pilot study by Huang et al.,18 human patients given either glutamine or a placebo who were undergoing radiation therapy were evaluated for the severity and duration of the mucositis experienced. The authors found that the glutamine-supplemented patients had less severe mucositis scores and a shorter duration of side effects.
Objectively, oral mucositis seen in our study was as severe as would be expected for this type of radiation treatment protocol. It is possible that the dose or dosing frequency of glutamine was inadequate or that local mucosal absorption instead of enteral absorption would be more beneficial. In the previously mentioned pilot study, patients were instructed to “swish and spit” to improve contact time and possible absorption at the oral site. Although 1 of 3 doses given to our patients was administered as an oral rinse while the patient was still anesthetized for radiation treatment, this “rinse and spit” type of dosing is not practical in veterinary patients.
Tissue PGE2 concentrations increased after initiation of treatment in both groups. Although the amount of increase in tissue PGE2 was greater and statistically significant in the unsupplemented dogs, it was not statistically significantly different from the change that occurred in the supplemented dogs. Therefore, the effect of glutamine as an adjunct therapy in a clinical setting remains questionable. Still, the apparent differences in mean PGE2 increase between the 2 groups may suggest that glutamine supplementation was effective at blunting the increase in PGE2 expected in the oral mucosa of these patients but that the effect could not be detected statistically because of a low power of 33%. Glutamine is thought to influence PGE2 increasing glutathione levels.12 It is also possible that glutamine can augment the immune function by increasing activity of NK cells, which could modulate a local inflammatory response and possibly decrease PGE2.13 Measurement of glutathione in this study was not performed and may be necessary to further elucidate a possible cause and effect. Plasma PGE2 levels did not vary over time in the supplemented group and did not reflect the changes seen in the tissue. Most likely, the increases seen in the tissue are a profound local effect and do not influence whole-body PGE2 concentrations.
Contrary to our hypothesis, plasma glutamine concentrations were not significantly increased over baseline in the supplemented group. However, they did not decrease at the end of treatment time point as was seen in the unsupplemented group when inflammation and mucositis is at its peak (Fig. 4). Although these changes were not significantly different, it is possible that the oral glutamine supplementation prevented a drop in plasma, glutamine levels that would have occurred without supplementation. Possible reasons for a lack of increase over baseline include inadequate dose or an even greater need for glutamine by the body as a whole because of possible catabolic states associated with cancer and its treatment that could deplete the amino acid pool.
Limitations of this
study include small sample size and associated limited statistical power,
and lack of placebo control group. Unsupplemented dogs were used as
comparison, but not all values of interest were available in these patients.
In conclusion, oral glutamine supplementation is well tolerated in patients
receiving it in conjunction with radiation therapy. Additional studies
are essential using a placebo controlled design with adequate power
to see if routine clinical use is warranted to help decrease side effects
and the possible negative impact on quality of life.
References
1. Souba WW: Glutamine and cancer. Ann Surg 218:715–728, 1993.
2. Souba WW, Klimberg VS, Plumley DA, et al: The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res 8:383–391, 1990.
3. Souba WW. Glutamine: A key substrate for the splanchnic bed. Ann Rev Nutr 11:285–308, 1991.
4. Klimberg VS, Nwokedi E, Hutchins LF, et al: Glutamine facilitates chemotherapy while reducing toxicity. JPEN 16:83S–87S, 1992.
5. Klimberg
VS, Souba WW, Dolson DJ, et al: Prophylactic glutamine protects the
intestinal mucosa from radiation injury. Cancer 66:62–68, 1990.
6. Ziegler TR, Benfell K, Smith RJ, et al: Saftey and metabolic effects of L–glutamine administration in humans. JPEN 14:137S–146S, 1990.
7. Dechelotte P, Darmaun D, Rongier M, et al: Absorption and metabolic effects of enterally administered glutamine in humans. Am J Phys 260:G677–682, 1991.
8. Fox AD, Kripke SA, DePaula J, et al: Effect of a glutamine-supplemented enteral diet on methotrexate induced entercolitis. JPEN 12:325–331, 1988.
9. Anderson PM, Schroeder G, Skubitz KM: Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 83:1433–1439, 1998.
10. Kisseberth WC, MacEwen EG: Complications of cancer and its treatment. In SJ Withrow, EG MacEwan, eds: Small Animal Clinical Oncology, 2nd ed. pp. 129–146. Philadelphia: WB Saunders, 1996.
11. Ogilvie GK, Vail DM: Metabolic alterations and nutritional therapy for the veterinary cancer patient. In SJ Withrow, EG MacEwan, eds: Small Animal Clinical Oncology, 2nd ed. pp. 117–128. Philadelphia: WB Saunders, 1996.
12. Rouse K, Nwokedi E, Woodliff JE, et al: Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann Surg 221:420–426, 1995.
13. Klimberg VS, Kornbluth J, Cao Y, et al: Glutamine supresses PGE2 synthesis and breast cancer growth. J Surg Res 63:293–297, 1996.
14. Zicker SC, Rogers QR: Temporal changes in the concentrations of amino acids in plasma and whole blood of healthy neonatal foals from birth to two days of age. Am J Vet Res 55:1020–1029, 1994.
15. Zicker SC, Rogers QR: Use of plasma amino acid concentrations in the diagnosis of nutritional and metabolic diseases in veterinary medicine. In Proceedings IVth Congress of the International Society for Animal Clincal Biochemestry (J.J. Kaneko, ed) University of California, Davis, California, July 18–21, 1990.
16. Maclouf J, Grassi J, Pradelles P. Development of enzyme-immunoassay techniques for the measurement of eicosanoids. In Waldren TL, Hughes HN, eds: International Conference on Prostaglandin and Lipid Metabolism in Radiation Injury. Rockfield, MD, 1986; pp. 355–364. New York: Plenum Press, 1987.
17. Lana SE, Dernell WS, LaRue SM, et al: Slow release cisplatin combined with radiation for the treatment of canine nasal tumors. Vet Radiol 1997; 38: 474–478.
18. Huang EY, Leung SW, Wang CG, et al: Oral glutamine
to alleviate radiation-induced oral mucositis: a pilot randomized trial.
Int J Radiat Oncol Biol Phys 46:535–539,
2000.
Table 1.
Mucositis Scores
Grade/score
0 1 2
3 4
Mucous No change Injection, Patchy
mucositis Confluent fibrinous Ulceration,
Membrane over baseline +/– mild sign +/– inflammatory mucositis, hemorrhage
Appearance of discomfort
serosanguinous +/– discharge, severe pain
+/– moderate signs or
necrosis
of discomfort
| Figure 1. Oral mucositis scores for patients throughout treatment. Mean scores ± standard deviation are reported. | 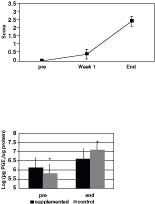 |
| Figure 2. Tissue PGE2 concentrations in both groups.
Mean scores ± standard deviation are reported. The symbol * denotes
a significant difference (P = .006). |
|
| Figure
3. Plasma bicycle PGE2 concentrations in supplemented dogs
throughout treatment. Mean scores ± standard deviation are reported. |
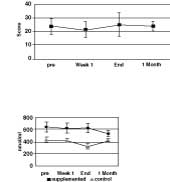 |
| Figure 4. Plasma glutamine concentrations
in both groups throughout treatment. Mean scores ± standard deviation
are reported. |
ISSN# 1542-2666