|
The INTERNATIONAL JOURNAL of APPLIED RESEARCH In Veterinary Medicine |
 |
| Current Issue |
| Previous Issues |
| Reprint Information |
| Back to The International Journal of Applied Research in Veterinary Medicine |
Effect of Vitamin
E on Glucose-6-Phosphate Dehydrogenase Enzyme Activity from Rainbow
Trout (Oncorhynchus mykiss) Erythrocytes in vitro and in vivo
O. Hisar†
S. Beydemir‡
I. Gülçin‡
S. ArasHisar
T. Yanik†
†
‡Atatürk University, Arts and Science Faculty,
Department of Chemistry, Erzurum, Turkey
KEY WORDS: Glucose-6-phosphate dehydrogenase, rainbow
trout, erythrocyte,
vitamin E
Abstract
In this study, the effect of vitamin E on rainbow trout (Oncorhynchus mykiss) erythrocyte glucose-6-phosphate dehydrogenase (G6PD) enzyme activity was investigated in vitro and in vivo. For this purpose, G6PD enzyme was purified from rainbow trout erythrocyte using ammonium sulphate precipitation and 2´, 5´-ADP Sepharose 4B affinity gel chromatography, a simple and rapid method. The G6PD enzyme was obtained with a specific activity of 16.7 EU/mg proteins and 1,852-fold in a yield of 60.6%. A temperature of 4˚C was maintained during the purification process. To asses the purity of the enzyme, SDS polyacrylamide gel electrophoresis was performed, and its electrophoretic pattern showed a single band. In vitro studies showed that vitamin E activated G6PD enzyme. In vivo studies showed that the activity of the control, which did not contain vitamin E, was determined as 7.59 ± 1.92 EU (g Hb)-1. The activities of groups after the vitamin E injection were measured at 1, 3, and 5 hours, and corresponding activities were found to be 3.12 ± 0.34, 8.37 ± 1.02, and 5.87 ± 2.18 EU (g Hb)-1. In light of in vivo experiments, we understood that vitamin E statistically inhibited the G6PD enzyme activity (P < .05) after the first hour following vitamin E injections.
Introduction
Vitamin E is the major lipid soluble antioxidant present in blood and cell membranes of higher organisms and plays a critical role in preventing lipid peroxidation. Vitamin E can donate a hydrogen atom to a lipid peroxyl radical to generate lipid hydroperoxide, thus terminating the progression of lipid peroxidation.1 Other functions of vitamin E have also been proposed, for example, in maintaining membrane protein thiols,2 in stabilizing membrane structure,3 in preventing certain diseases,4 in activating fish immune functions,5,6 and in protecting cells with other antioxidants from damage and lysis induced by oxidative stress.7 Oxidative stress refers to the disturbance of the equilibrium between antioxidants and pro-oxidants towards oxidants. During oxidative stress, many adverse effects are triggered, including lipid peroxidation, protein oxidation, and interference with cellular homoeostasis, which can lead to cell death.4
Glucose 6-phosphate dehydrogenase (D-glucose 6-phosphate: NADP+ oxidoreductase EC 1.1.1.49; G6PD) catalyzes the first and rate-limiting reaction of the pentose phosphate metabolic pathway, which is a unique source for NADPH synthesis with concomitant reduction of NADP+ in cells.8-10 NADPH production is decreased in G6PD deficiency.11 At the cellular level a continuous supply of reducing equivalents in the form of NADPH is essential to growth and proliferation process, serving as they do as hydrogen and electron sources for a variety of reductive biosynthetic reactions.12 In addition, NADPH also participates in cell-membrane protection and cell detoxification from xenobiotics through the gluthatione reductase-peroksidase system and the mixed-function oxidases.13-15
Vitamin E is natural component of the diet, and its concentrations can be easily changed without altering the energenic metabolism. Moreover, it can be administered in high doses at low cost. Although, the influence of vitamin E administration on stress parameters have been extensively investigated,16-19 studies related to the effects of this application on enzyme inhibition or activation are unfortunately rare. Therefore, the goal of this study was to determine the inhibition effects of vitamin E on enzyme activities of G6PD, which improves the total antioxidative defense capacity of the organism from rainbow trout erythrocyte, in vitro and in vivo.
Materials and Methods
Chemicals
For this study, 2’, 5’-ADP Sepharose 4B was purchased from Pharmacia (Uppsala, Sweden). NADP+, glucose 6-phosphate, protein assay reagent, chemicals for electrophoresis, and vitamin E were purchased from Sigma Chemical Company (Steinheim, Germany). All other chemicals used were of analytical grade and obtained from either Sigma or Merck (Darnstadt, Germany).
Fish Maintenance and Feeding
The rainbow trout (n = 20) used for the purification of G6PD enzyme from erythrocytes were 1 year old (mean weight, 200 ± 20 g). The average water temperature was 9˚ ± 2˚C; dissolved oxygen was 8 to 9 ppm; pH was 7.8; and total hardness was 102 mg as CaCO3 during the tests. At the time of sample collection, fish were fed a commercial pelleted trout feed 2 times at 1% body weight per day.20
Preparation of the Hemolysate
After rainbow trout
were anesthetized (MS-222 was used), blood was sampled from the caudal
vein using a 10-mL heparinized (5 IU mL-1) plastic syringe and then
transferred into heparin-containing tubes. These samples centrifuged
at 2,500 x g for 15 minutes. The plasma was removed by drip. After the
packed red cells were washed with KCl solution (0.16 M) 3 times, the
samples were centrifuged at 2,500 x g each time, and supernatants were
removed. The erythrocytes were hemolyzed with 5 volume of ice-cold water
and centrifuged (4˚C, 10,000 ¥ g) for 30
minutes to remove the ghosts and intact cells.21
Ammonium Sulphate Fractionation
and Dialysis
Hemolysate was brought among 40% to 65% (NH4)2SO4 saturation with solid (NH4)2SO4. The precipitate was separated by centrifugation at 5,000 g for 15 minutes and dissolved in a small amount of 50 mM phosphate buffer (pH 7.0). The enzyme solution was dialyzed at 4˚C in 50 mM K-acetate/5m M K-phosphate buffer (pH 7.0) for 2 hours, with 2 changes of buffer.22,23
2’, 5’-ADP Sepharose 4B
Affinity Chromatography
For 10 mL of bed volume, 2 g of dry 2’, 5’-ADP Sepharose
4B was washed several times in 400 mL of distilled water. With several
washings, the impurities were removed and the gel conditioned. After
removal of the air in the gel, it was resuspended in the buffer (0.1
M K-acetate + 0.1 M K-phosphate, pH 6.0) with a ratio of 25% buffer
and 75% gel and was packed in a column (1 x 10 cm). After precipitation
of the gel, it was equilibrated with the same buffer by means of a peristaltic
pump (flow rate: 50 mL/h). The dialyzed enzyme solution obtained previously
was loaded on the column, and the flow rate was adjusted to 20 mL/h.
Then the column was sequentially washed with 25 mL of 0.1 M K-acetate
+ 0.1 M K-phosphate, (pH 6.0) and 25 mL 0.1 M K-acetate + 0.1 M K-phosphate
(pH: 7.85). The washing with 0.1M KCl + 0.1M K-phosphate, (pH 7.85)
was continued until the final absorbance difference became 0.05. Finally,
the enzyme was eluted with the solution of 80 mM K-phosphate + 80 mM
KCl + 0.5 mM NADP+ + 10 mM EDTA
(pH: 7.85). The enzyme activity was measured in final fractions, and
the activity-containing tubes were collected together. The protein was
determined in the resultant solution. During all procedures, the temperature
was kept at 4˚C.22,24
Activity Determination
The enzymatic activity was measured by Beutler’s method.25 One enzyme unit was defined as the enzyme amount reducing 1 µmol NADP+ per 1 minute.
Protein Determination
Quantitative protein
determination was spectrophotometrically measured at 595 nm according
to Bradford’s method,26 with bovine serum albumin being used as a standard.
SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE)
To control enzyme purity, Laemmli’s procedure27 was performed in 3% and 10% acrylamide concentrations containing 10% SDS for running and stacking gel, respectively. The gel was stabilized in the solution containing 50% propanol + 10% TCA + 40% distilled water for 30 minutes. The staining was made for about 2 hours in the solution of 0.1% Coommassie Brillant Blue R-250 + 50% methanol + 10% acetic acid. Finally, the washing was performed in the solution of 50% methanol + 10% acetic acid + 40% distilled water until protein bands were cleared.
In vitro Experiment for Vitamin E
The effect of vitamin E on G6PD enzyme activity was investigated for addition to the enzyme activity determination medium (total volume, 1 mL) in 5 different final concentrations (0.012, 0.024, 0.048, 0.072, and 0.096 mM). Enzyme activity was determined by the Beutler method25 and repeated 3 times. Activity (EU/mL) values of G6PD enzyme for 5 different final concentrations of vitamin E were drawn by using regression analysis graphs on computer.
In vivo Study for Vitamin E
In
this study, 10 rainbow trout (250 ± 24 g) were selected for vitamin
E and control groups. First, fish were anesthetized in water containing
tricaine methane sulfonate (MS-222, 1/10000), and 0.5-mL blood samples
from the control group were collected from the caudal sinus using heparinized
syringes and placed into a heparinized vacutainer. For the vitamin E
group, 10 mg kg-1 vitamin E was injected into the muscle around the
dorsal fin.28 Blood samples were taken from each trout at 1, 3, and
5 hours after injection. All blood samples were centrifuged at 2,500
g, then the erythrocyte pellet was washed with 0.16 M KCl 3 times, and
the supernatant was discarded. One volume from the erythrocyte pellet
result was hemolyzed in 5 volumes of ice water. As a result, hemolysate
was prepared. Studies were performed at 4˚C. G6PD activity was
assayed using the Beutler method.25 A one-way analysis of variance (ANOVA)
followed by Duncan’s multiple range test was used to determine significant
difference among G6PD activity means at 0, 1, 3, and 5 hours.
Results
In the purification procedures, at the hemolysate; total volume was 100 mL with 3,500 mg total protein and a 0.009 EU/mg protein-specific activity. The yield was assumed as 100% at this stage. At ammonium sulphate precipitation (40% to 65%), total volume was 24 mL with 410.4 mg total protein, a 0.056 EU/mg protein-specific activity, and 69.1% yield. Finally, at 2´, 5´- ADP Sepharose 4B affinity chromatography stage, total volume was 8 mL, with 1.2 mg total protein, a 16.67 EU/mg protein-specific activity, and 60.6% yield. After hemolysate preparation, ammonium sulphate precipitation and affinity gel chromatography, the enzyme was purified 1,852-fold (Table 1). To control the purity of enzyme, SDS polyacrylamide gel electrophoresis was used, and its electrophoretic pattern showed a single band (Fig. 1).
In vitro studies showed that vitamin E showed an activation effect on G6PD (Fig. 2). After this experiment, vitamin E was also studied in vivo (Table 2). We observed that the activity of the control group, which does not contain any vitamin E, was 7.59 ± 1.92 EU (g Hb)-1. Then vitamin E injection was performed, and the activities of the groups after vitamin E injection were measured at 1, 3, and 5 hours. The corresponding activities are, respectively, 3.12 ± 0.34, 8.37 ± 1.02, and 5.87 ± 2.18 EU (g Hb)-1. The significant (P < .05) inhibition was found in 1 hour after injection. Thus, it was determined that in vivo studies related to vitamin E did not support in vitro studies.
Discussion
Many chemicals affect the metabolism of biota at relatively low dosages by altering normal enzyme activity. With some of these interactions, a high reactivity is seen, involving a high degree of inhibition of a specific enzyme, which accounts for the effect on the whole animal or plant.29
In the present study, vitamin E showed an activation effect on the rainbow trout erythrocyte G6PD enzyme activity in vitro but a significant (P < .05) decrease in the rainbow trout erythrocyte G6PD enzyme activity 1 hour after alpha tocopherol injection was also observed in vivo. Thus, in vivo studies related to vitamin E did not support the results of in vivo studies. The possible explanation for this situation would be the applied pharmacologic dosage of vitamin E used in the in vivo study. Because the vitamin E concentration used in vivo was 10 mg/kg, which may be excessive for fish, although it is reported as the normal dose for mammals.28,30 It may have been too much greater than that of the in vitro study.
The inhibitory effect of vitamin E on G6PD enzyme activity in vivo affects the pentose phosphate pathway, which is the only source of NADPH in erythrocytes. G6PD is the first enzyme in the pentose phosphate pathway. The major role of NADPH in erythrocyte is in the regeneration of reduced glutathione (GSH), which preserves the integrity of red blood cell membrane sulfhdryl groups and detoxifies hydrogen peroxide and oxygen radicals in and on the red blood cells.31,32 Thus, the inhibition of G6PD enzyme activity may be cause an increase in oxidative stress. Consequently, dosage, duration, and administration methods of vitamin E should be researched further, and excessive use of this vitamin should be avoided
References
1. Niki E: Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr 54:119S–1124S, 1991.
2. Takenaka Y, Miki M, Yasuda H, Mino M: The effect of a-tocopherol as an antioxidant on the oxidation of membrane protein thiols induced by free radicals generated in different sites. Arch Biochem Biophys 285:344–350, 1991.
3. Urano S, Inomori Y, Sugawara T, et al: Vitamin E: inhibition of retinol-induced hemolysis and membrane-stabilizing behaviour. J Biol Chem 267:18365–18370, 1992.
4. Lii C-K, Chen H–W, Wang S–T: Inhibition of protein thiol modification in hepatocytes isolated from rats supplemented with vitamin E under oxidative stress. Toxicol In Vitro 10:557–566, 1996.
5. Anderson DP: Immunostimulants, adjuvants, and vaccine carriers in fish: Applications to aquaculture. Ann Rev Fish Dis 2:281–307, 1992.
6. Sakai M: Current research status of fish immunostimulants. Aquaculture 172:63–92, 1999.
7. Konar V, Yilmaz Ö, Öztürk AI, Kirba S, Arslan M: Antimicrobial and biological effects of bomphos and phomphos on bacterial and yeast cells. Bioorg Chem 28:214–225, 2000.
8. Beydemir S, Kulaçoiglu DN, Çiftçi M, Küfrevioiglu ÖI: The effects of some antibiotics on sheep lens glucose 6-phosphate dehydrogenase in vitro. Eur J Ophthalmol 13:155–161, 2003.
9. Bianchi D, Bertrant O, Haupt K, Coello N: Effect of gluconic acid as a secondary carbon source on non-growing L-lysine producers cells of Corynebacterium glutamicum. Enzyme Microb Tech 28:754–759, 2001.
10. Kuo W, Lin J, Tang TK: Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms nih 3t3 cells and induces tumors in nude mice. Int J Cancer 85:857–864, 2000.
11. Beutler E: Glucose-6-phosphate dehydrogenase deficiency. Blood 84:3613–3636, 1994.
12. Walzem RL, Storebakken T, Hung SSO, Hansen RJ: Relationship between growth and selected liver enzyme activities of individual rainbow trout. J Nutr 121:1090–1098, 1991.
13. Barroso JB, Peragón J, García-Salguero L, et al: Variations in the kinetic behaviour of the NADPH-production systems in different tissues of the trout when fed on an amino-acid-based diet at different frequencies. Int J Biochem Cell B 31:277–290, 1999.
14. Corpas FJ, Barroso JB, Sandalio LM, et al: A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem J 330:777–784, 1998.
15. Díez-Fernández C, Sanz N, Cascales M: Changes in glucose-6-phosphate dehydrogenase and malic enzyme gene expression in acute hepatic injury induced by thiochetamide. Biochem Pharmacol 51:1159–1163, 1996.
16. Fletcher TC: Dietary effects on stress and health. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB, eds: Fish Stress and Health in Aquaculture. Cambridge: Society for Experimental Biology, 1997.
17. Mulero V, Esteban MA, Meseguer J: Effects of in vitro addition of exogenous vitamins C and E on gilthead seabream (Sparus aurata L.) phagocytes. Vet Immunol Immunop 66:185–199, 1999.
18. Ortuño J, Esteban MA, Cuesta A, Meseguer J: Effect of oral administration of high vitamin C and E dosages on the gilthead seabream (Sparus aurata L.) innate immune system. Vet Immunol Immunop 79:167–180, 2001.
19. Ortuño J, Esteban MA, Meseguer J: The effect of dietary intake of vitamins C and E on the stress response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immun 14:145–156, 2003.
20. Hisar O, Yanik T, ArasHisar S: Clinical and pathological investigation of Psychrobacter immobilis infection in rainbow trout (Oncorhynchus mykiss, Walbaum). Isr J Aquacult-Badmid 54:186–196, 2002.
21. Bülbül M, Hisar O, Beydemir S, et al: The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhibit Med Chem 18:371–375, 2003.
22. Ninfali P, Orsenigo T, Barociani L, Rapa S: Rapid purification of glucose-6-phosphate dehydrogenase from mammal’s erythrocyte. Prep Biochem 20:297–309, 1990.
23. Çiltas A, Erdogan O, Hisar O, Çiftçi M: Effects of chloramine–T and CuSO4 on enzyme activity of glucose 6-phosphate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Isr J Aquacult– Bamid 55:187–196, 2003.
24. Beydemir S, Çiftçi M, Küfrevioilu ÖI: Purification and characterization of glucose 6-phosphate dehydrogenase from sheep erythrocytes and inhibitory effects of some antibiotics on enzyme activity. J Enzyme Inhibit Med Chem 17:271–277, 2002.
25. Beutler E: Red cell metabolism manual of biochemical methods. London: Academic Press, 1971.
26. Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254, 1976.
27. Laemmli DK: Clevage of structural proteins during in assembly of the head of bacteriophage T4. Nature 227:680–683, 1970.
28. Plack PA, Bieri JG: Mtabolic products of I-tocopherol in the livers of rats given inraperitoneal injections of [14C]-I-tocopherol. Biochim Biophys Acta 84:729–738, 1964.
29. Çiftçi M, Küfrevioiglu ÖI, Gündoigdu M, Özmen I: Effects of some antibiotics on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 41:109–113, 2000.
30. Çiftçi M, Bilici D, Küfrevioiglu ÖI: Effects of melatonin on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 44:7–11, 2001.
31. Deutsch J: Glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, Bergmeyer J, eds: Methods of enzymatic analysis. Berlin: Verlagsgerellschaff, 1983.
32. Weksler BB, Moore A, Tepler J: Hematology. In:
Andreoli TE, Carpenter CCJ, Plum F, Smith LH, eds: Cecil
essentials of medicine. Philadelphia: WB Saunders Company, 1990.
Figure 1. SDS-PAGE bands of G6PD. Lane 1: Standard
proteins; yeast hexokinase (100 kDa), rabbit heart creatine phosphokinase
(81 kDa), bovine serum albumin (66 kDa), bovine liver glutamic dehydrogenase (55 kDa), bovine spleen deoksiribonuclease
(38 kDa); Lanes 2 and 3 are rainbow trout G6PD.
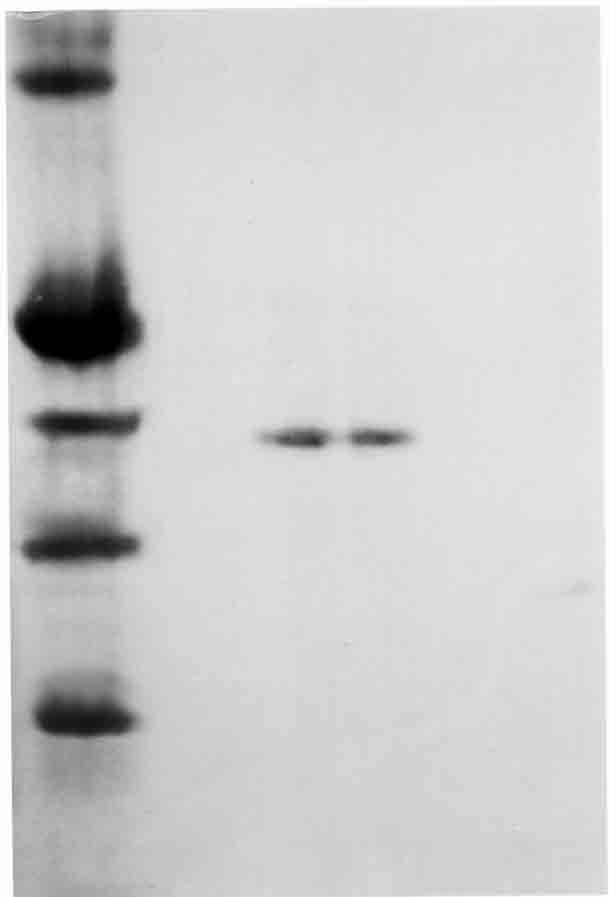
Figure 2. The in vitro effects of vitamin E on glucose-6-phosphate
dehydrogenase enzyme activity of rainbow trout erythrocyte.
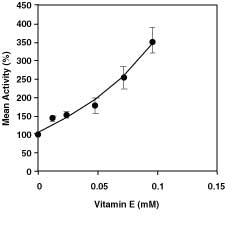
Table 1. Purification Scheme
of Glucose-6-Phosphate Dehydrogenase from Rainbow Trout Erythrocytes
Total Total Total
Specific
Purification Activity Volume Protein Protein Activity Activity
Yield Purification (EU/mL) (mL) (mg/mL)
(EU) (mg)
(EU) (EU/mg)
(%) factor
Hemolysate
0.33 100
35 3500
33 0.009
100 1
Ammonium
0.95 24
17.10 410.40
22.80 0.056
69.1 6.22
sulphate
precipitation
(40%–65%)
2´,
5´- ADP 2.50 8
0.15 1.20
20 16.667
60.6 1,852
Sepharose 4B
affinity
chromatography
Table 2.
Statistical Values Obtained From in vivo
Studies for Vitamin E
Compound Time Mean ± SD
EU (g Hb)-1
Vitamin E
Control 7.59 ± 1.92a
1h 3.12 ± 0.34b
3h 8.37 ± 1.02a
5h 5.87 ± 2.18ab
ISSN# 1542-2666